Abstract
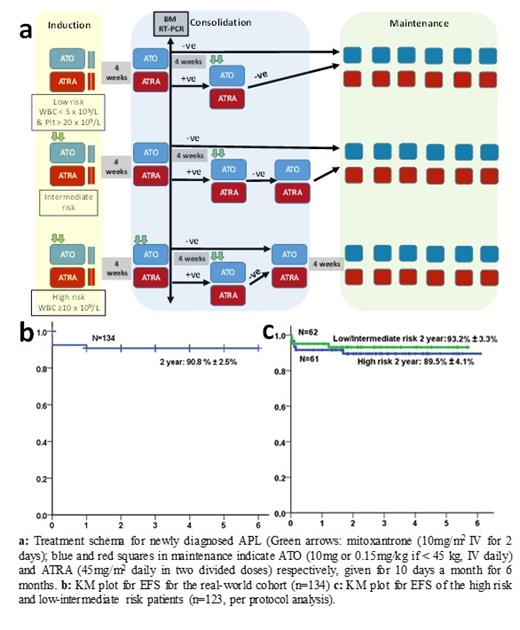
In the setting of clinical trials, high cure rates have been reported for acute promyelocytic leukemia (APL) with the chemotherapy-free combination of arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) for low-intermediate risk disease, and with the addition of gemtuzumab ozogamicin or minimal anthracycline for high-risk disease. Despite clear in-vitro synergy between ATO and ATRA, most clinical trial protocols in APL have not used these drugs concurrently beyond induction. There is limited real world data on the use of chemotherapy-free combination of ATO and ATRA with minimal anthracycline use in the treatment of APL, especially in the high risk group. We did a retrospective analysis of the clinical outcomes of patients with newly diagnosed APL treated at our center from January 2015 to May 2020 using concurrent ATO, ATRA and minimal anthracycline in a risk stratified manner. The data was frozen and analyzed as on 1st June 2021. During the study period, 167 patients were diagnosed to have APL at our hospital. Of these, we excluded 5 patients who had presented with relapsed disease and 28 patients who were treated with single agent ATO. The remaining 134 patients were treated with a uniform protocol as summarized in Figure 1a. Figure 1b shows the KM plot for EFS (2 year: 90.8±2.5%) for these 134 patients. For analysis of regimen safety and efficacy, we excluded patients who wished to pursue treatment elsewhere within the first 1 week of treatment (n=7), and patients who had severe infections or life-threatening bleeding at presentation or before therapy initiation and subsequently died (n=4). Thus, a total of 123 patients with newly diagnosed APL were included for further analysis. The median age was 35 years (IQR: 24 to 45 years). Forty-six (37.4%) were females. The median duration of symptoms prior to admission was 14 days (IQR: 7 to 28 days). Sixty-one (49.6%) had high risk APL while the remaining 62 (50.4%) had low-intermediate risk APL. During induction, 28 (22.7%) patients had major bleeding while 5 (4%) patients developed major thrombosis. Sixty-seven (55%) patients had at least one documented infection during induction therapy. The median number of packed red cell concentrates, fresh frozen plasma, platelet rich concentrates and cryoprecipitates transfused during induction therapy was 5 (IQR: 3 to 6.5), 10 (IQR: 3 to 30), 40 (IQR: 23 to 55) and 6 (IQR: 0 to 18) respectively. Nineteen (15.4%) patients developed differentiation syndrome; all were treated with steroids, 8 required transient cessation of treatment and 6 required intensive care. Eight (6.5%) patients died during induction. During induction, grade 3 hepatotoxicity was noted in 8 (6.5%) patients, 10 (8.1%) patients had ATRA related headache or benign intracranial hypertension of which 5 required transient cessation of ATRA, while 11 (8.9%) patients had transient QTc prolongation of which 6 required transient cessation of ATO. Seventeen (13.8%) patients developed symptomatic sensory neuropathy requiring treatment. None required permanent discontinuation of therapy. At a median follow up of 854 days, there were 2 (1.6%) hematologic relapses and no deaths beyond induction therapy. The 2-year OS and EFS for the cohort (n=123) are 93.4% ± 2.3% and 91.6% ± 2.6% respectively (Figure 1c). Two (1.6%) patients have developed second malignancies. In a real world setting, concurrently administered ATO and ATRA with minimal anthracycline use results in excellent long term survival, even in the high risk group. Early deaths due to delayed presentation with infections and life threatening bleeding remain an unmet need for future research along with the need for strategies to reduce treatment-related toxicities.
Mathews: Christian Medical College: Patents & Royalties: US 2020/0345770 A1 - Pub.Date Nov.5, 2020; AML: Other: Co-Inventor.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal